


PhD Institution: National Centre for Biological Science, India Post PhD Institution: Max Planck Institute of Molecular Cell Biology and Genetics, Germany
Many RNA and RNA binding proteins form dynamic, phase-separated, membrane-less condensates in cells which often act as reaction centres in the cells. They can form in minutes and also dissolve as soon as the function is over. Importantly, these condensates form solid-like aggregates in neurodegeneration diseases. We try to understand the role of RNA in the formation, properties and function of the condensates in the cells.
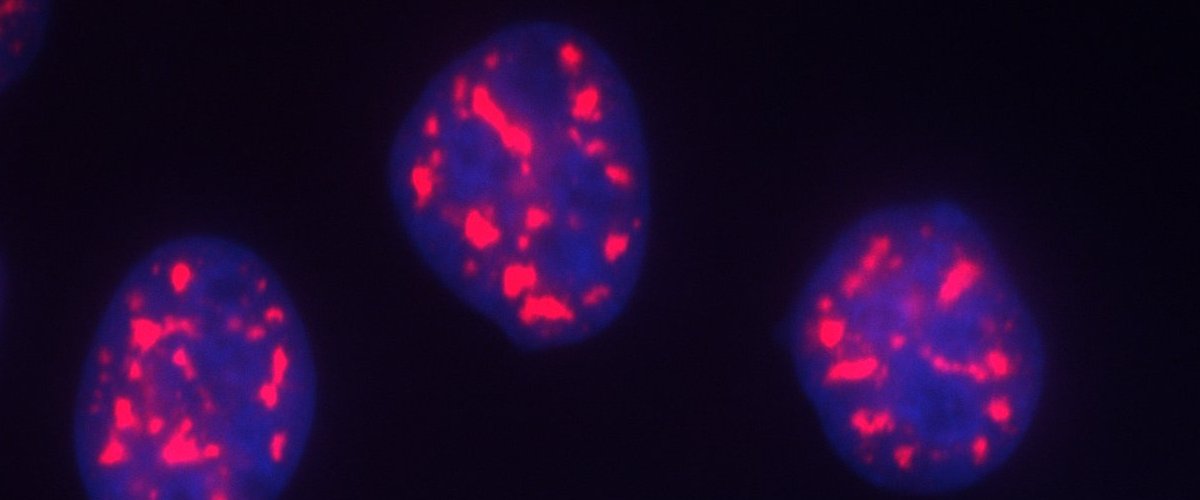
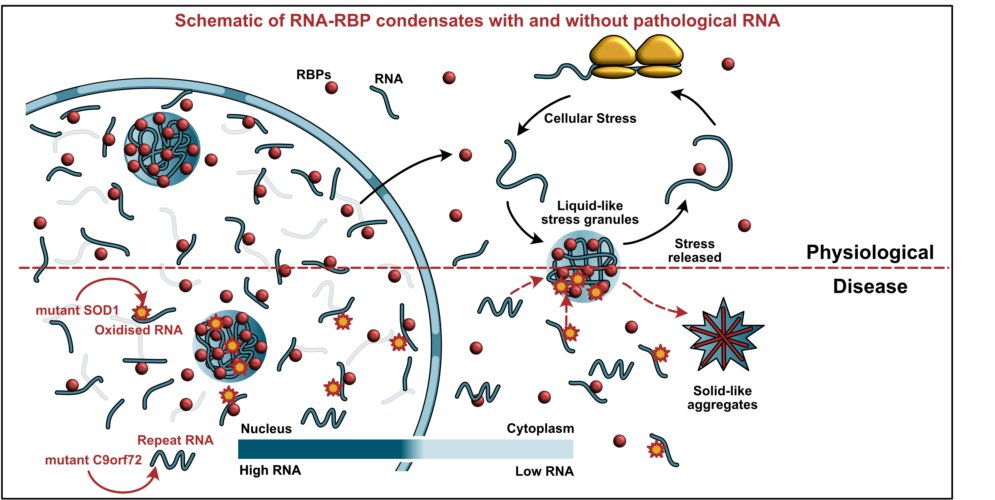
Cellular microenvironment is compartmentalized into biomolecular condensates of RNA binding proteins (RBPs) and RNA, like nuclear-splicing speckles, DNA repair sites and cytoplasmic stress granules. These condensates are dynamic which exchange components with the surrounding, behave like liquid droplets and are formed by a physicochemical process known as liquid-liquid phase separation. Changes in phase behaviour of condensates are associated with pathology in many neurodegenerative conditions. We focus on understanding the role of RNA interaction in the formation and function of biomolecular condensates.
The phase separation of the biomolecular condensates is known to be driven by low complexity domain containing RBPs and condensate-specific scaffold RNA. In cells the RBPs are always associated with RNAs the mammalian cells are filled with RNAs, around 20-30 picogram of RNA per cell. We found that the nucleus has a very high concentration of RNA which keeps many neurodegeneration-associated, prion-like RBPs in a soluble state. This also explains why these proteins often aggregate in the cytoplasm on mislocalization to the cytoplasm. Neurodegeneration is also associated with ageing and inflammation. We found that the condensates can also sequester endogenous immunogenic RNA and buffer inflammation. Taken together we want to understand the condensates from a molecular perspective as well as at the cellular and systems level. Specifically we are addressing the following questions in the coming years:
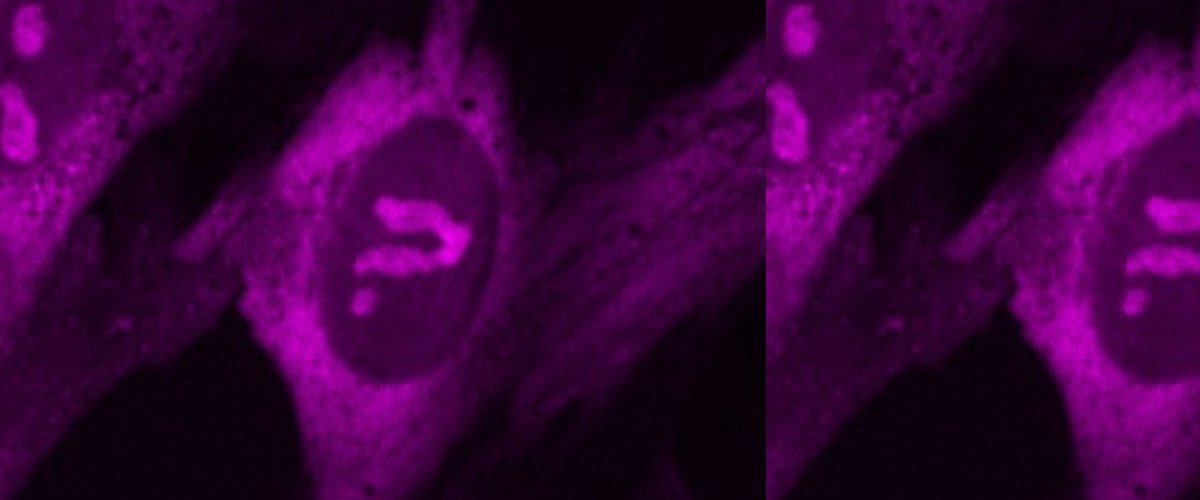
Seeing is believing and hence we develop in vitro and in cellular imaging modalities to map the dynamics of these condensates in regular fluorescence and super-resolution. We also explore these condensates in mammalin cell lines and in organismal tissue context.

Email : akankshasing@iisc.ac.in
Designation : Phd Student
Category : Molecular and Cellular Biology

Email : anuvar@iisc.ac.in
Designation : Phd Student
Category : Molecular and Cellular Biology

Email : nischithabaliga@gmail.com
Designation : Research Assistant
Category : Molecular and Cellular Biology

Email : padmanavad@iisc.ac.in
Designation : Phd Student
Category : Molecular and Cellular Biology

Email : prarthanag@iisc.ac.in
Designation : Phd Student
Category : Molecular and Cellular Biology

Email : prernanarwal@iisc.ac.in
Designation : Phd Student
Category : Molecular and Cellular Biology

Email : shobhae@iisc.ac.in
Designation : Postdoc
Category : Molecular and Cellular Biology

Email : sumerusugata@iisc.ac.in
Designation : Phd Student
Category : Molecular and Cellular Biology

Email : twinkal@iisc.ac.in
Designation : Postdoc
Category : Molecular and Cellular Biology
Fused in sarcoma (FUS) is an abundant RNA-binding protein, which drives phase separation of cellular condensates and plays multiple roles in RNA regulation. The RNA-binding ability of FUS protein is crucial to its cellular function. Here, our molecular simulation study on the FUS–RNA complex provides atomic resolution insights into the observations from biochemical studies and also illuminates our understanding of molecular driving forces that mediate the structure, stability, and interaction of the RNA recognition motif (RRM) and RGG domains of FUS with a stem–loop junction RNA. We observe clear cooperativity and division of labor among the ordered (RRM) and disordered domains (RGG1 and RGG2) of FUS that leads to an organized and tighter RNA binding. Irrespective of the length of RGG2, the RGG2–RNA interaction is confined to the stem–loop junction and the proximal stem regions. On the other hand, the RGG1 interactions are primarily with the longer RNA stem. We find that the C terminus of RRM, which make up the “boundary residues” that connect the folded RRM with the long disordered RGG2 stretch of the protein, plays a critical role in FUS–RNA binding. Our study provides high-resolution molecular insights into the FUS–RNA interactions and forms the basis for understanding the molecular origins of full-length FUS interaction with RNA.
C-terminal variants in CDC42 encoding cell division control protein 42 homolog underlie neonatal-onset cytopenia, autoinflammation, rash, and hemophagocytic lymphohistiocytosis (NOCARH). Pyrin inflammasome hyperactivation has been shown to contribute to disease pathophysiology. However, mortality of NOCARH patients remains high despite inflammasome-focused treatments. Here, we demonstrate in four NOCARH patients from three families that cell-intrinsic activation of type I interferon (IFN) is a previously unrecognized driver of autoinflammation in NOCARH. Our data show that aberrant innate immune activation is caused by sensing of cytosolic nucleic acids released from mitochondria, which exhibit disturbances in integrity and dynamics due to CDC42 dysfunction. In one of our patients, treatment with the Janus kinase inhibitor ruxolitinib led to complete remission, indicating that inhibition of type I IFN signaling may have an important role in the management of autoinflammation in patients with NOCARH.
Recognition of pathogen-derived foreign nucleic acids is central to innate immune defense. This requires discrimination between structurally highly similar self and nonself nucleic acids to avoid aberrant inflammatory responses as in the autoinflammatory disorder Aicardi-Goutières syndrome (AGS). How vast amounts of self RNA are shielded from immune recognition to prevent autoinflammation is not fully understood. Here, we show that human SAM-domain- and HD-domain-containing protein 1 (SAMHD1), one of the AGS-causing genes, functions as a single-stranded RNA (ssRNA) 3′exonuclease, the lack of which causes cellular RNA accumulation. Increased ssRNA in cells leads to dissolution of RNA-protein condensates, which sequester immunogenic double-stranded RNA (dsRNA). Release of sequestered dsRNA from condensates triggers activation of antiviral type I interferon via retinoic-acid-inducible gene I-like receptors. Our results establish SAMHD1 as a key regulator of cellular RNA homeostasis and demonstrate that buffering of immunogenic self RNA by condensates regulates innate immune responses.
Prion-like RNA binding proteins (RBPs) such as TDP43 and FUS are largely soluble in the nucleus but form solid pathological aggregates when mislocalized to the cytoplasm. What keeps these proteins soluble in the nucleus and promotes aggregation in the cytoplasm is still unknown. We report here that RNA critically regulates the phase behavior of prion-like RBPs. Low RNA/protein ratios promote phase separation into liquid droplets, whereas high ratios prevent droplet formation in vitro. Reduction of nuclear RNA levels or genetic ablation of RNA binding causes excessive phase separation and the formation of cytotoxic solid-like assemblies in cells. We propose that the nucleus is a buffered system in which high RNA concentrations keep RBPs soluble. Changes in RNA levels or RNA binding abilities of RBPs cause aberrant phase transitions.
Proteins such as FUS phase separate to form liquid-like condensates that can harden into less dynamic structures. However, how these properties emerge from the collective interactions of many amino acids remains largely unknown. Here, we use extensive mutagenesis to identify a sequence-encoded molecular grammar underlying the driving forces of phase separation of proteins in the FUS family and test aspects of this grammar in cells. Phase separation is primarily governed by multivalent interactions among tyrosine residues from prion-like domains and arginine residues from RNA-binding domains, which are modulated by negatively charged residues. Glycine residues enhance the fluidity, whereas glutamine and serine residues promote hardening. We develop a model to show that the measured saturation concentrations of phase separation are inversely proportional to the product of the numbers of arginine and tyrosine residues. These results suggest it is possible to predict phase-separation properties based on amino acid sequences.
Stress granules (SG) are membrane‐less compartments involved in regulating mRNAs during stress. Aberrant forms of SGs have been implicated in age‐related diseases, such as amyotrophic lateral sclerosis (ALS), but the molecular events triggering their formation are still unknown. Here, we find that misfolded proteins, such as ALS‐linked variants of SOD1, specifically accumulate and aggregate within SGs in human cells. This decreases the dynamics of SGs, changes SG composition, and triggers an aberrant liquid‐to‐solid transition of in vitro reconstituted compartments. We show that chaperone recruitment prevents the formation of aberrant SGs and promotes SG disassembly when the stress subsides. Moreover, we identify a backup system for SG clearance, which involves transport of aberrant SGs to the aggresome and their degradation by autophagy. Thus, cells employ a system of SG quality control to prevent accumulation of misfolded proteins and maintain the dynamic state of SGs, which may have relevance for ALS and related diseases.
RNA-protein (RNP) granules have been proposed to assemble by forming solid RNA/protein aggregates or through phase separation into a liquid RNA/protein phase. Which model describes RNP granules in living cells is still unclear. In this study, we analyze P bodies in budding yeast and find that they have liquid-like properties. Surprisingly, yeast stress granules adopt a different material state, which is reminiscent of solid protein aggregates and controlled by protein disaggregases. By using an assay to ectopically nucleate RNP granules, we further establish that RNP granule formation does not depend on amyloid-like aggregation but rather involves many promiscuous interactions. Finally, we show that stress granules have different properties in mammalian cells, where they show liquid-like behavior. Thus, we propose that the material state of RNP granules is flexible and that the solid state of yeast stress granules is an adaptation to extreme environments, made possible by the presence of a powerful disaggregation machine.

aishwarya.juneja012@gmail.com

amruthagowda778@gmail.com

aqsaellahie2772@gmail.com

soundaryask2000@gmail.com
Date: 2025-06-30
Qualification: Master’s /4 years Bachelor Degree
Position: JRF
POSTED-01.03.2025
Applications are invited from suitable candidates for available positions of CSIR-Aspire Junior Research Fellows (1 position) at Dr. Shovamayee MCB, Indian Institute of Science. More details of the projects can be obtained from the website and laboratories.
ESSENTIAL QUALIFICATIONS:
Junior Research Fellow: Master’s /4 years Bachelor Degree in any biology/bioengineering stream with good academic record (Minimum of 60% aggregate). General age limit is 28years with relaxation of 3 years for OBC-NCL and 5 years for SC/ST, PwD, Third gender, and female candidates.
EMOLUMENTS: As per CSIR project norms.
DURATION: Positions are available for maximum of three months. The initial appointment will be for 3 months and will be extended only after a performance evaluation. As with all project positions, the position will be co-terminus with the end of the project.
Interested candidates may send applications with a copy of their resume, one page letter of intent and photocopies of credentials & experience (if any) on or before 5:00 PM on 30th June, 2025.